April 28, 2025
2 min learn
Key takeaways:
- Immune checkpoint inhibitor-based mixtures are the popular first-line remedy for metastatic clear cell renal cell carcinoma.
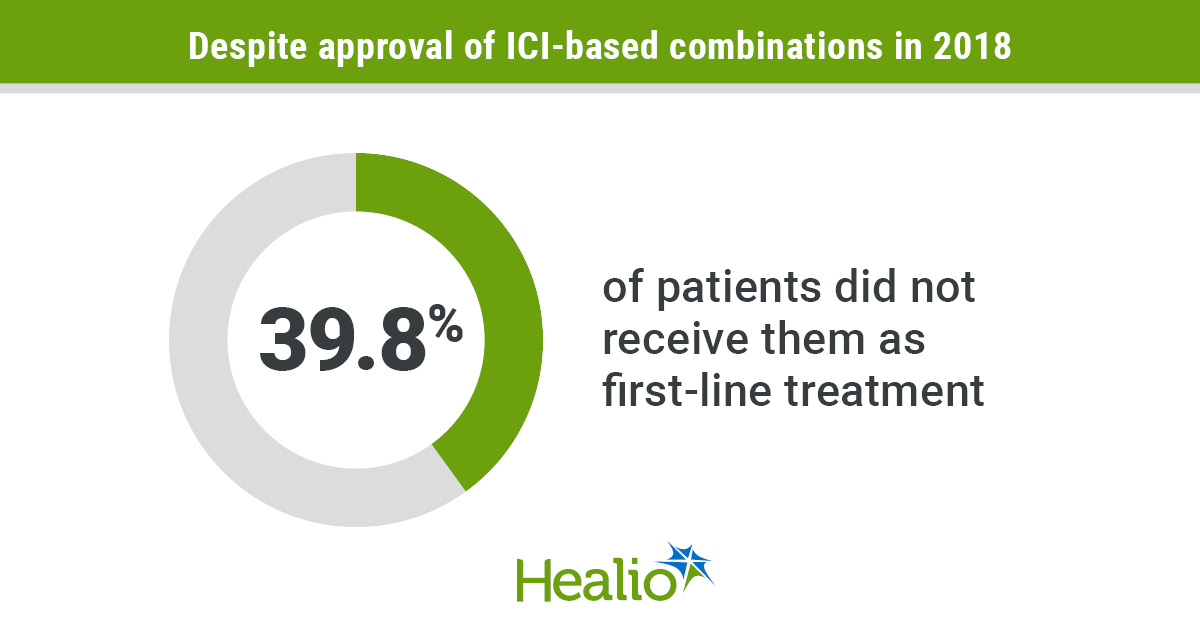
- Nonetheless, greater than a 3rd of sufferers usually are not receiving them.
Immune checkpoint inhibitor-based mixtures have turn out to be the popular first-line remedy for sufferers with metastatic clear cell renal cell carcinoma, however greater than a 3rd of eligible sufferers don’t obtain them, examine outcomes confirmed.
“The FDA can approve [kidney cancer] therapies and tips can change, however sufferers nonetheless aren’t getting therapies which can be permitted,” researcher Umang Swami, MD, MS, assistant professor of oncology at College of Utah’s Huntsman Most cancers Institute, informed Healio. “We wished to have a look at the precise uptake in a U.S.-based, real-world affected person inhabitants and decide whether or not we are literally adopting new remedy suggestions per present tips.”

Knowledge derived from Ozay ZI, et al. JAMA Netw Open. 2025;doi:10.1001/jamadermatol.2025.1201.
Remedy for metastatic clear cell renal cell carcinoma (ccRCC) has modified considerably because the approval of immune checkpoint inhibitor (ICI)-based mixtures. Nonetheless, knowledge on their use earlier than and after regulatory approval stay restricted, in keeping with examine background.
Swami and colleagues carried out a nationwide cohort examine to look at remedy patterns and attrition charges over time on this affected person inhabitants.
The researchers utilized digital well being report knowledge from roughly 280 most cancers clinics within the U.S. to establish sufferers with metastatic ccRCC who obtained first-line remedy between Jan. 1, 2011, and Jan. 20, 2023. They excluded sufferers receiving systemic remedy for at the very least two malignant neoplasms and people enrolled in medical trials.
Their evaluation included 8,534 sufferers (median age, 66 years; interquartile vary, 59-74; 70.7% males; 71% white).
The researchers used April 16, 2018 — the date of the primary ICI-based mixture approval — as a reference level and divided sufferers into two cohorts primarily based on after they initiated remedy.
The primary cohort, which started remedy earlier than the reference date, included 4,561 sufferers (53.4%). The second cohort, which started remedy after the reference date, included 3,973 sufferers (46.6%).
Earlier than regulatory approval of ICI-based mixtures, most sufferers (78.8%) obtained first-line tyrosine kinase inhibitor monotherapy.
After approval of ICI-based mixtures, a majority (60.2%) of sufferers obtained them within the first-line setting. In each time intervals, TKI monotherapy remained the most typical therapies used within the second-line setting.
Analyses of remedy attrition earlier than and after approval of ICI-based mixtures confirmed the share of sufferers who obtained second-line remedy (57.9% vs. 37.6%) and third-line remedy (31.9% vs. 14.1%) declined considerably approval the reference date.
Researchers acknowledged examine limitations, together with its retrospective design and lack of sure endpoint knowledge as a result of reliance on EHRs. In addition they famous that knowledge don’t account for an affiliation between remedy choice throughout strains of remedy and baseline demographics.
The findings present many sufferers don’t obtain first-line ICI-based mixtures, regardless of the prevalence they’ve demonstrated vs. TKI monotherapy. This means a “have to optimize remedy choice by implementing present tips in medical observe,” Swami and colleagues wrote.
“These knowledge inform us that we at the moment solely have a number of mechanisms to assault the illness,” Swami informed Healio. “We’d like totally different mechanisms by which we are able to assault kidney most cancers and, on the similar time, we would like these to be much less poisonous and extra out there and quicker performing. These are among the issues that can truly make a distinction.”
For extra data:
Umang Swami, MD, MS, may be reached at umang.swami@hci.utah.edu.