Need to keep on high of the science and politics driving biotech in the present day? Join to get our biotech publication in your inbox.
Good morning. We’ve received a busy earnings day in the present day, so let’s get straight into it.
Tariff questions swirl round pharma earnings
Right here’s what a number of massive drugmakers stated about tariffs when reporting earnings this morning:
Merck made a small downward adjustment to the highest of its earnings steerage for the rest of the yr, and stated it expects $200 million in extra prices associated to tariffs imposed by the U.S. on imports from different nations, and by international nations, largely from China, on U.S. items.
Bristol Myers Squibb raised its 2025 income and earnings steerage, and stated its revised forecast included the impression on U.S. merchandise shipped to China, however doesn’t account for any potential pharmaceutical sector tariffs.
In the meantime, Roche and Sanofi confirmed their outlooks for the yr, even with the tariff uncertainty.
François Roger, Sanofi’s finance chief, advised reporters the corporate has been gaming out the impression of the levies. “We’ve got run all eventualities,” he stated.
Roche CEO Thomas Schinecker offered some extra particulars on the way it’s making an attempt to get forward of the potential duties.
The Swiss pharma agency decided that simply 4 of its medicines made up greater than 90% of its potential tariff publicity, Schinecker advised reporters on a name. Three of them had been already produced within the U.S., and the corporate has been ramping up its manufacturing of the medication there. The fourth had not beforehand been made within the U.S., so the corporate needed to first do some tech switch work, however it’s now scaling up the manufacturing of the drug stateside. Schinecker didn’t disclose what the 4 medication are.
The corporate has additionally been increase its stock in each the U.S. and China.
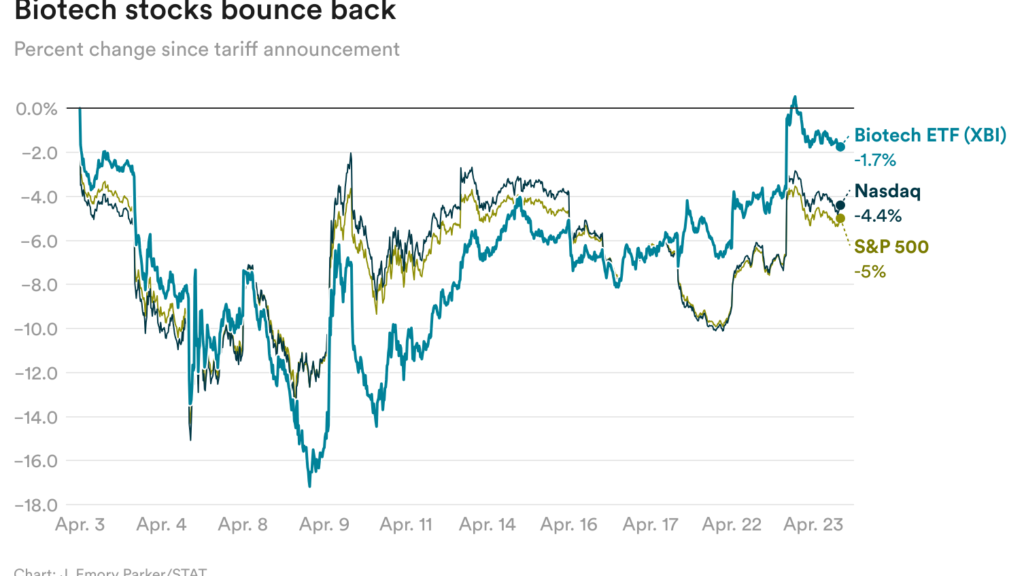
Biotech shares bounce again (for now)
Biotech shares surged the previous two days, in a uncommon, and what could also be transient, upturn for the trade that’s been battered by uncertainties round tariffs and drug regulation.
They rose, together with the broader inventory market, as President Trump appeared to melt his tone on the commerce conflict with China. The Wall Avenue Journal reported yesterday that the White Home is contemplating reducing the tariffs it’s imposed on Chinese language imports, in some instances by greater than half.
(The drug trade depends closely on uncooked components from China.)
Biotech shares at the moment are nearly again to the place they had been earlier than Trump’s “Liberation Day” announcement. However the president’s subsequent transfer on tariffs is, in fact, unpredictable, and it’s too early to breathe simple but.
My colleague Adam Feuerstein — who has been understandably a bit doom and gloom these days — has extra ideas on the view from Wall Avenue right here.
Novavax additionally bounces again
Among the many largest market winners yesterday was Novavax, whose shares soared over 16% after it stated it believes its Covid-19 vaccine is approvable, primarily based on latest conversations with the FDA.
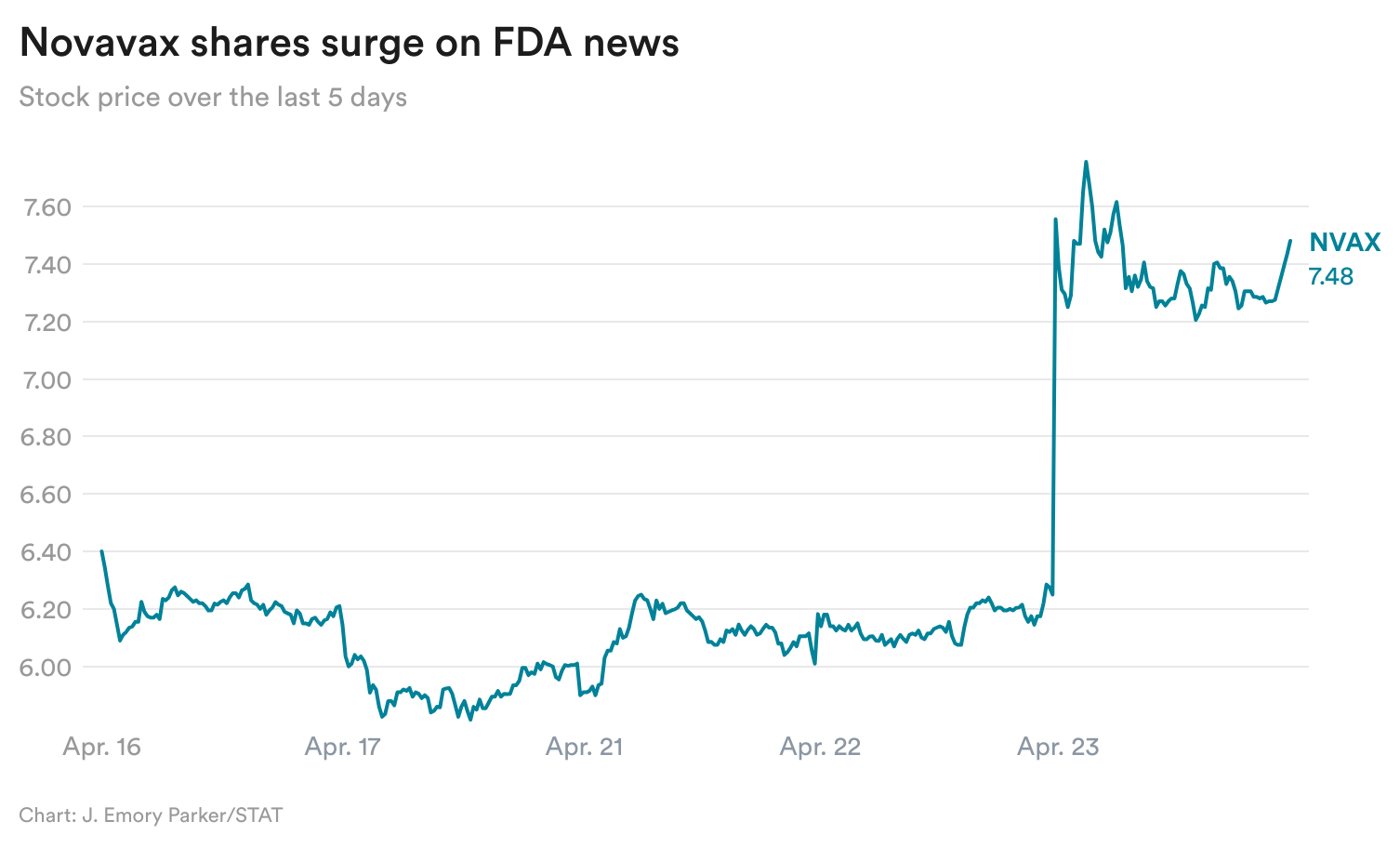
An FDA choice on the vaccine was initially anticipated on April 1, however was delayed. Politico beforehand reported {that a} high company official paused the approval course of for the vaccine to ask for extra information, elevating considerations about political interference in scientific assessments on the company.
Flagship’s new startup will develop ‘preemptive’ medication
Flagship Pioneering introduced this morning that it’s invested $50 million to create a brand new startup targeted on creating what it calls “preemptive medicines” to guard folks’s well being earlier than they get sick.
The startup, referred to as Etiome, has a platform that goals to forecast how people are more likely to progress into illnesses, outline illness phases, after which develop medication that may halt or reverse illnesses earlier than bodily signs start or long-term injury happens.
If the time period preemptive drugs sounds unfamiliar to you, that’s doubtless as a result of it’s not broadly utilized in Western drug improvement and well being care. The idea traces again to Japan. Flagship asserts that it’s totally different from preventative drugs, which stops a illness from ever occurring.
The agency, which invests in and creates biotechs, purchased into the preemptive drugs thought a couple of years in the past and employed former FDA fee Stephen Hahn to guide the group that created Etiome.
By no means having to expertise extreme illness would, in fact, be superb. However this type of effort raises the identical kinds of questions as preventative remedies — how a lot do we have to use these medication, which will be expensive and carry uncomfortable side effects, in populations who aren’t but sick?
Extra reads